Pelareorep in Gastrointestinal Cancers
For the growing number of patients with gastrointestinal cancers, effective therapies that improve treatment outcomes and meaningfully extend survival are limited.1 Indeed, fewer than 12% of patients receiving the current standard-of-care for pancreatic cancer survive 5 years beyond their diagnosis.2 To address this profoundly unmet need, Oncolytics is conducting the GOBLET trial to examine the potential of pelareorep and the checkpoint inhibitor atezolizumab in addition to standard-of-care therapy, where appropriate, for gastrointestinal cancers, including advanced/metastatic pancreatic cancer. The combination of pelareorep, gemcitabine, nab-paclitaxel, and atezolizumab received FDA Fast Track designation for the treatment of advanced/metastatic
pancreatic cancer.
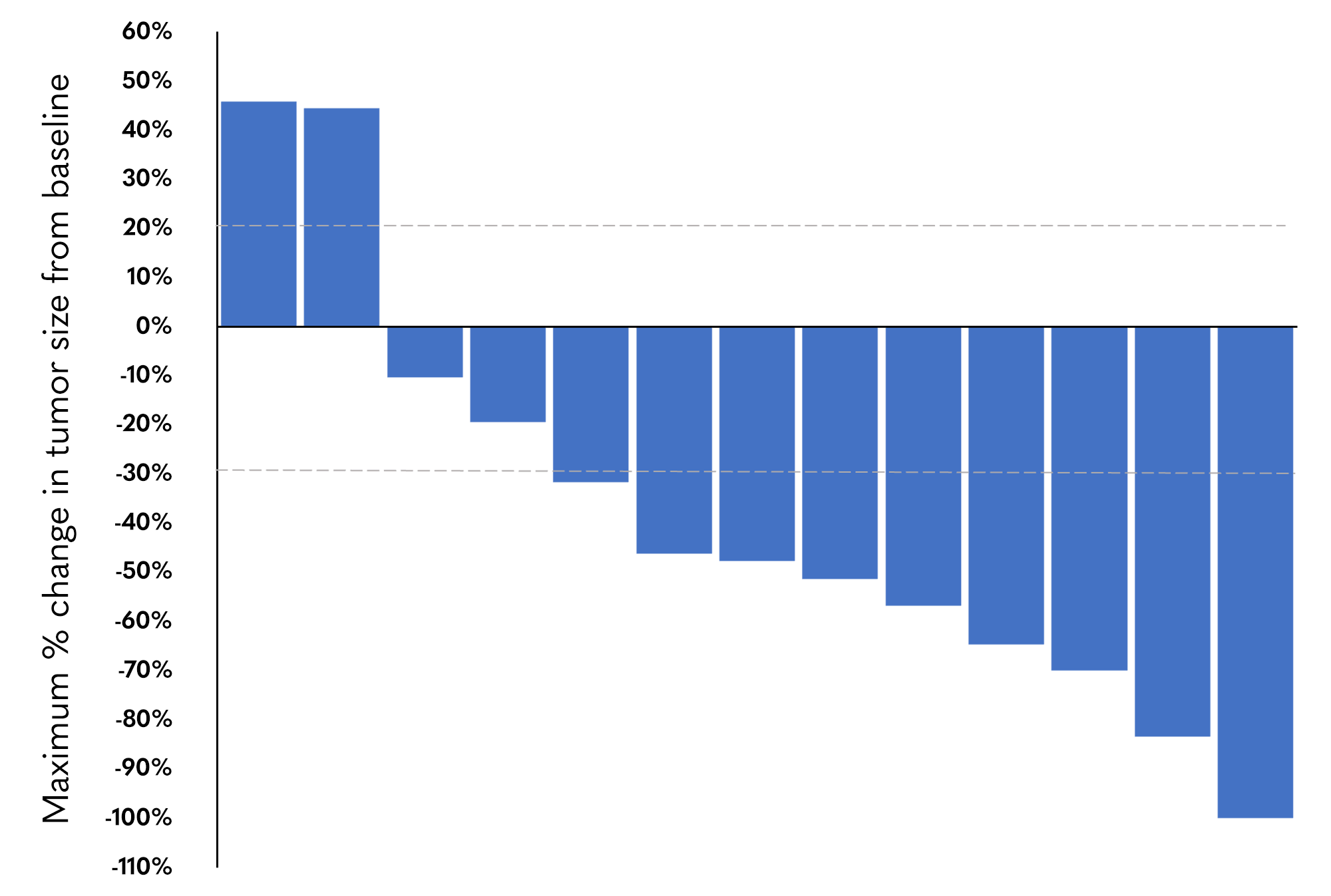
The 62% objective response rate from GOBLET Cohort 1, evaluating pelareorep in combination with gemcitabine, nab-paclitaxel, and atezolizumab in first-line metastatic pancreatic ductal adenocarcinoma, is more than double the response rate seen in historical control trials.3-6 As a result of the Fast Track designation and the promising data reported to date, Oncolytics is working with key opinion leaders and collaborative groups on the next stages of development.
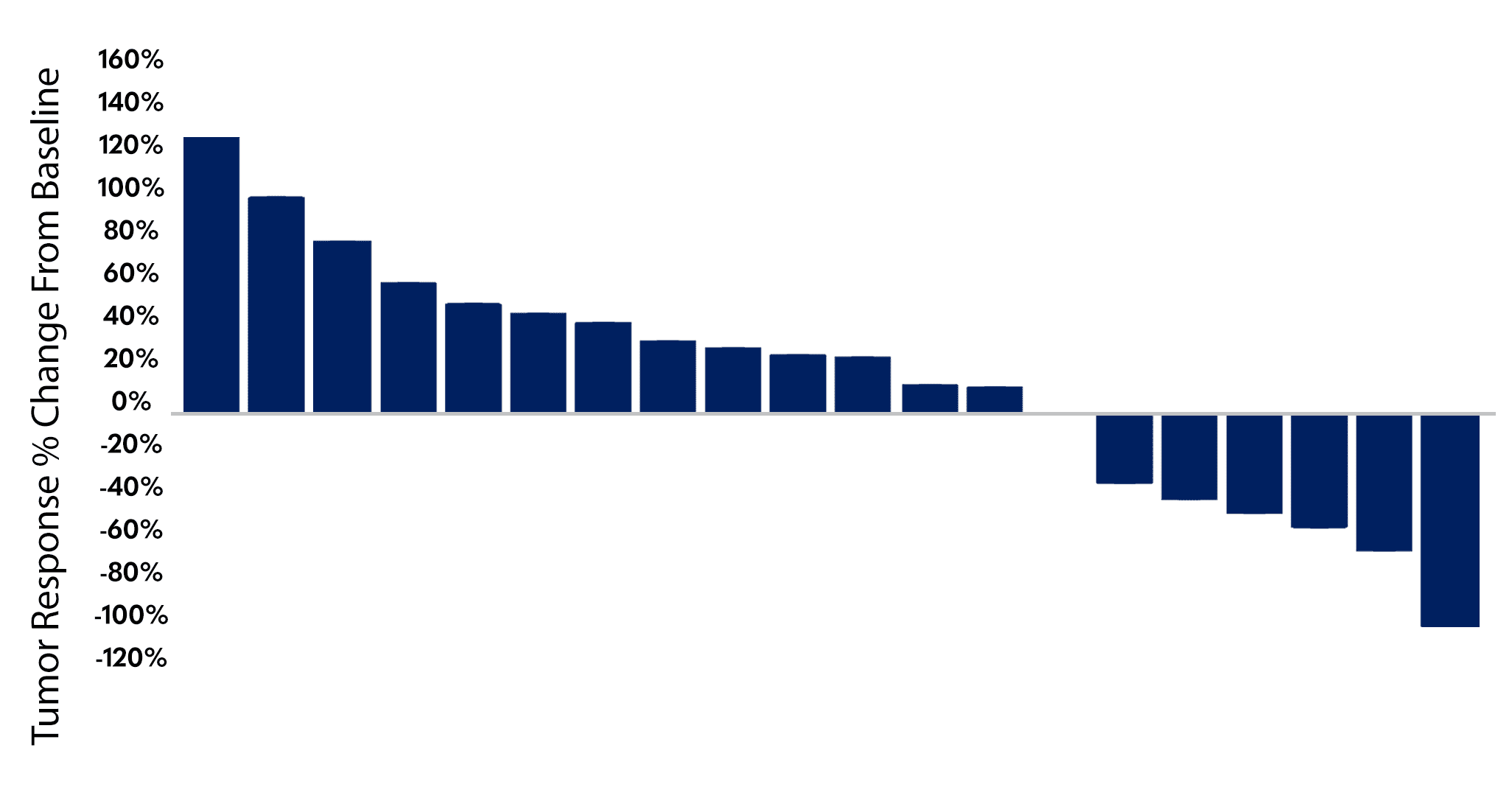
In second-line or later unresectable squamous cell carcinoma of the anal canal pelareorep and atezolizumab showed a 30% objective response rate, including two durable complete responses, a meaningful improvement compared to historical control trials.7-9 Oncolytics intends to engage the FDA to discuss a potential single-arm study designed for accelerated approval in second-line or later SCAC, with a potential launch date for such a study expected in the first half of 2026.
- Hakuno SK, Michiels E, Kuhlemaijer EB, Rooman I, Hawinkels LJAC, Slingerland M. Multicellular Modelling of Difficult-to-Treat Gastrointestinal Cancers: Current Possibilities and Challenges. Int J Mol Sci. 2022 Mar 15;23(6):3147. doi: 10.3390/ijms23063147. PMID: 35328567; PMCID: PMC8955095.
- National Cancer Institute. (2021). Cancer Stat Facts: Pancreatic Cancer. SEER Program, Surveillance, Epidemiology, and End Results. Retrieved April 5, 2023, from https://seer.cancer.gov/statfacts/html/pancreas.html
- Von Hoff D et al. N Engl J Med 2013; 369:1691-1703 DOI: 10.1056/NEJMoa1304369
- O’Reilly et al. Eur J Cancer. 2020 June; 132: 112–121. DOI:10.1016/j.ejca.2020.03.005
- Karasic et al. JAMA Oncol. 2019 Jul 1; 5(7):993-998. DOI: 10.1001/jamaoncol.2019.0684
- Tempero et al. Ann Oncol. 2021 May; 32(5):600-608. DOI: 10.1016/j.annonc.2021.01.070
- Rao S, et al. Phase II study of retifanlimab in patients (pts) with squamous carcinoma of the anal canal (SCAC) who progressed following platinum-based chemotherapy. Annals of Oncology. 2020 September. doi: https://doi.org/10.1016/j.annonc.2020.08.2272
- Marabelle A, et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol Hepatol. 2022 May;7(5):446-454. doi: 10.1016/S2468-1253(21)00382-4.
- Lonardi S, et al. Randomized phase II trial of avelumab alone or in combination with cetuximab for patients with previously treated, locally advanced, or metastatic squamous cell anal carcinoma: the CARACAS study. J Immunother Cancer. 2021 November;9(11):e002996. doi: 10.1136/jitc-2021-002996. PMID: 34815354; PMCID: PMC8611452.
GOBLET Publications
Learn more about how pelareorep remodels tumor microenvironments to enable immune access while training the immune system to recognize and kill tumor cells in patients with pancreatic and other GI cancers.
Contact Us
For questions regarding our science, partnership opportunities, or other inquiries, we encourage you to reach out.