Pelareorep for HR+/HER2- Metastatic Breast Cancer
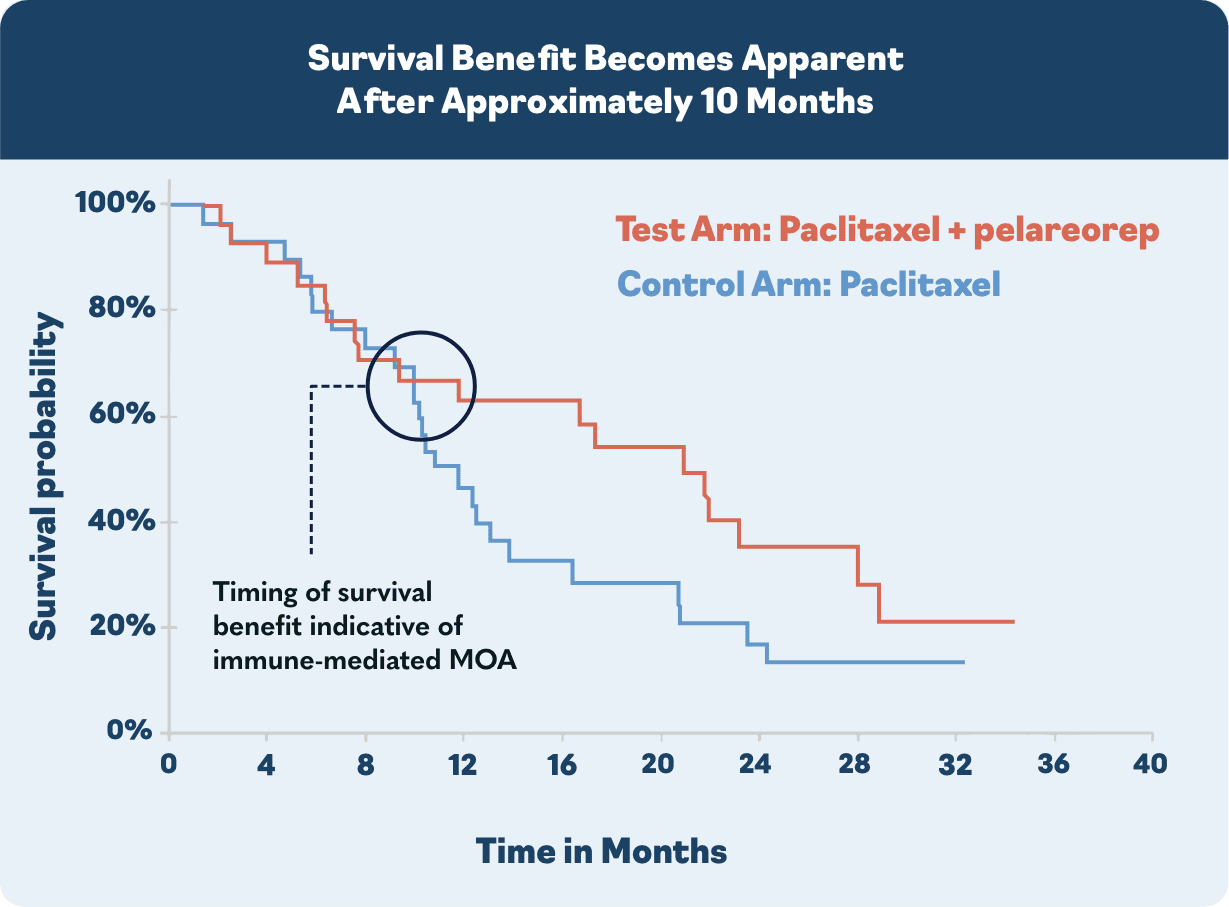
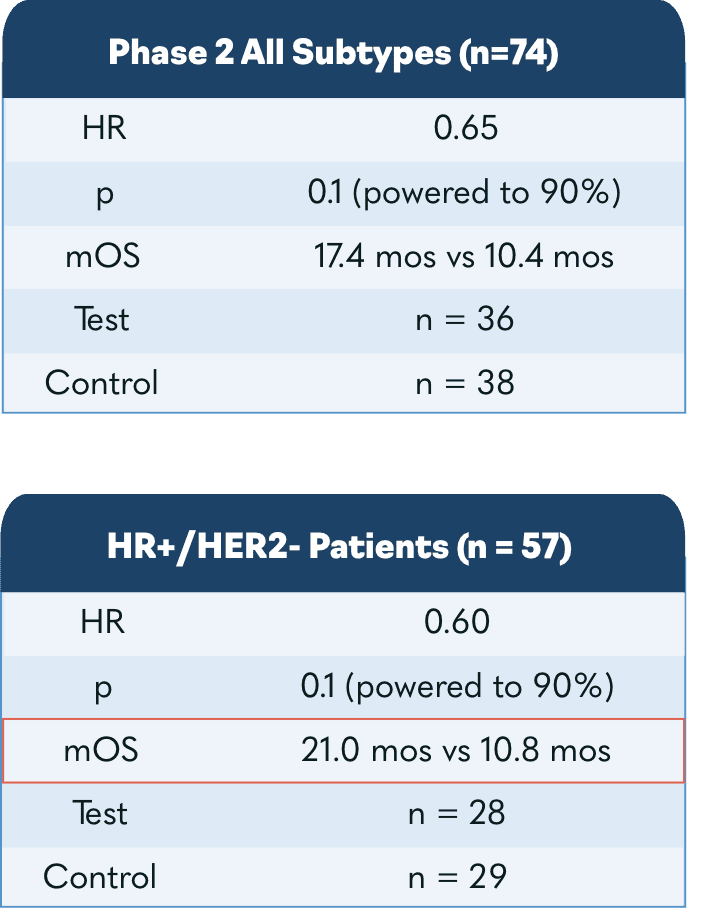
In the US, major European markets, and Japan, drug-treatable HR+/HER2- breast cancer cases are projected to rise to nearly 300,000 patients by 20281. To address this growing need, Oncolytics investigated pelareorep in a randomized Phase 2 clinical trial called BRACELET-1. The combination of pelareorep and the chemotherapy paclitaxel provided an estimated 14-month overall survival benefit compared to paclitaxel monotherapy. Progression-free survival was nearly double for pelareorep + paclitaxel compared to paclitaxel.
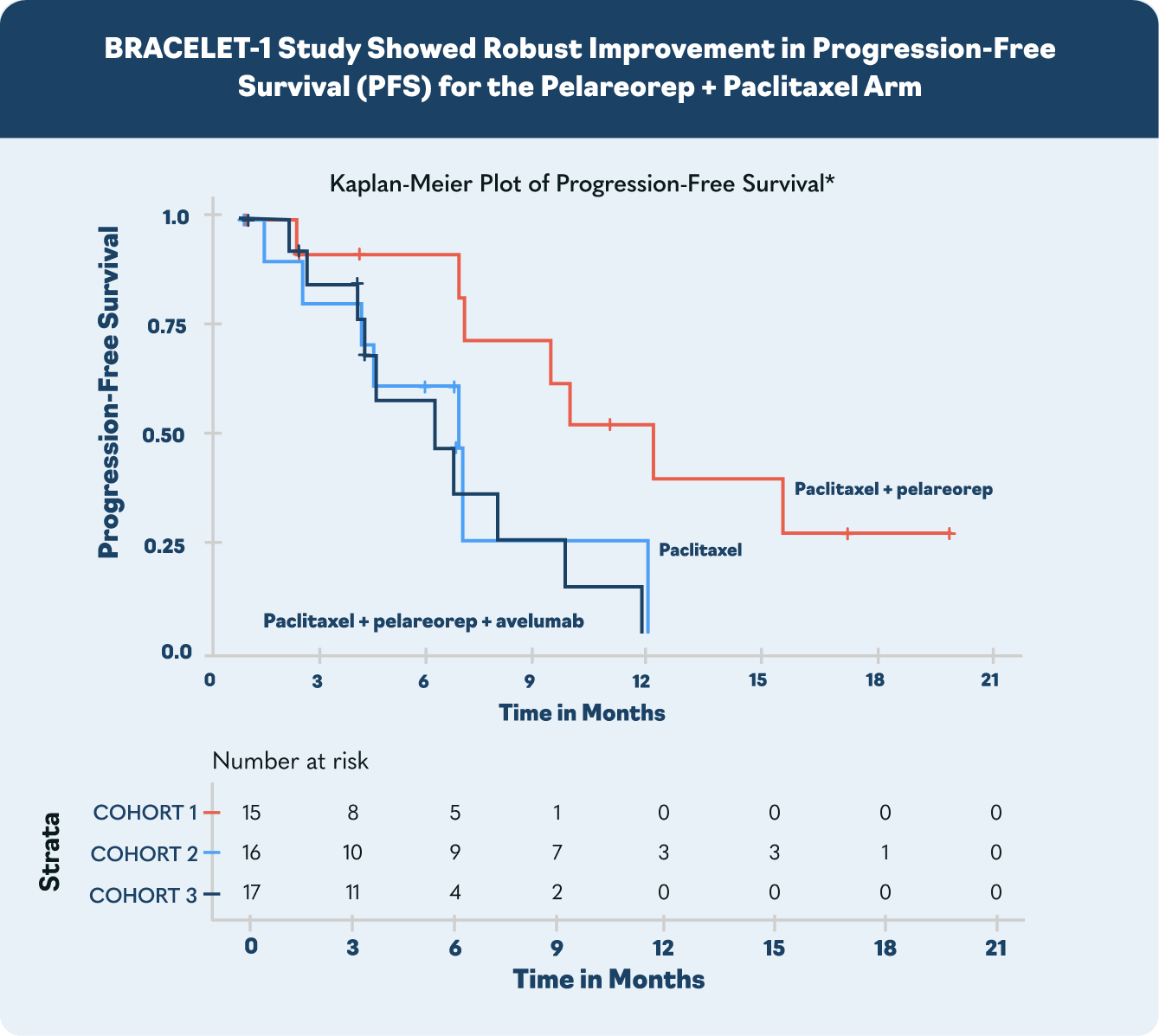
*Progression-free survival is defined as the time from randomization to the first documented disease progression per RECIST v1.1 or death from any cause, whichever occurs first.
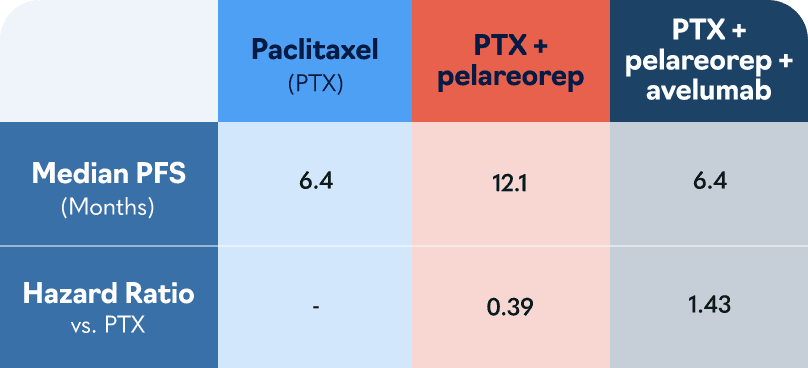
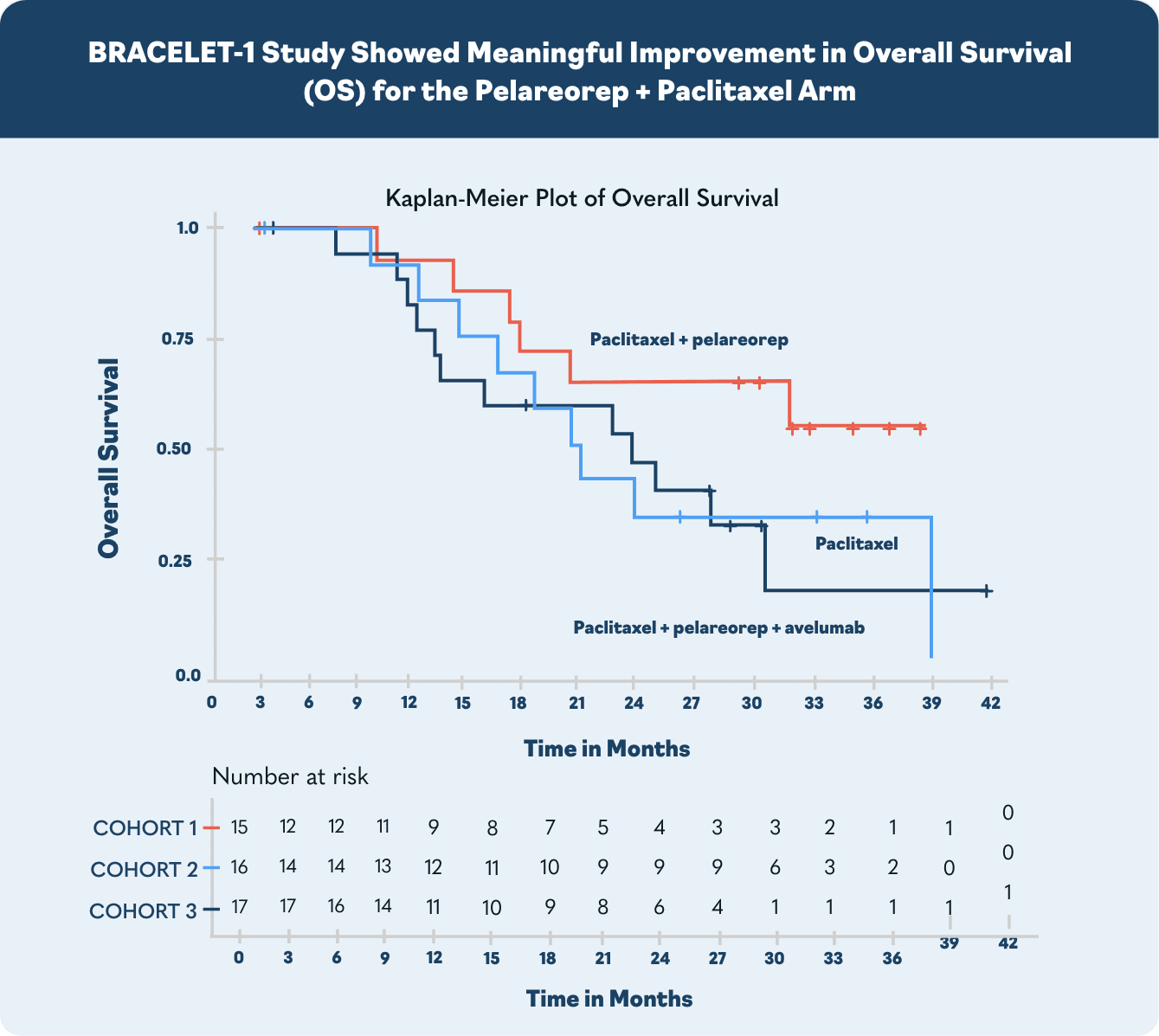
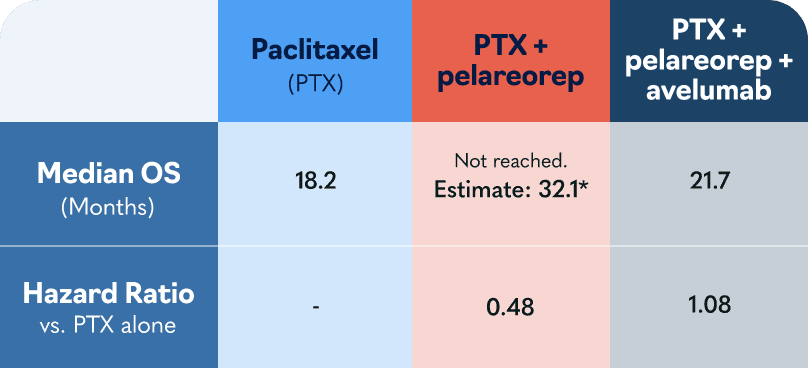
*The estimate assumes patients survived only until the next per protocol follow-up in 4 months. Had the patients survived only one day past their last respective follow-up the estimated median OS would be 28.7 months
In our IND-213 trial, treatment with pelareorep in combination with paclitaxel led to a statistically significant near doubling of overall survival in patients with HR+/HER2- metastatic breast cancer in a randomized setting.
- Clarivate Analytics. “Biopharma Breast Cancer Landscape & Forecast Disease”. Accessed February 28, 2023.
https://clarivate.com/products/research-reports/report/dlsfon0001-biopharma-breast-cancer-landscape-forecast-disease/.
Metastatic breast cancer
We have now treated over 100 HR+/HER2- metastatic breast cancer patients across two randomized studies and received productive feedback from the FDA. Our next step is to advance a two-arm registration-enabling study with a PFS primary endpoint that, depending on patient outcomes, will be powered to support a submission for accelerated approval with the FDA.
For additional details about this study, please visit the BRACELET-1 clinical trial page.
BRACELET-1 Publications
In the IND-213 study, the combination of pelareorep and paclitaxel nearly doubled overall survival in patients with HR+/HER2- metastatic breast cancer.
BRACELET-1 News
For the latest updates on the BRACELET-1 trial, click the link below.
Contact Us
For questions regarding our science, partnership opportunities, or other inquiries, we encourage you to reach out.